LYON, FRANCE (November 14th, 2025) – Nova In Silico, (“Nova”), a health-tech company pioneering digital twins in healthcare with its unique causal AI jinkōⓇ platform supporting teams with decision-ready insights, announced today that it prospectively predicted the magnitude and trajectory of LDL‑cholesterol (LDL‑C) reduction reported in Merck’s Phase 3 CORALreef Lipids study of the investigational oral PCSK9 inhibitor Enlicitide Decanoate (formerly MK‑0616). Nova’s predictions were publicly time‑stamped on Zenodo (https://doi.org/10.5281/zenodo.16925767) ahead of the readout at AHA Scientific Sessions 2025 and were performed entirely independently— without contact with Merck and without access to embargoed data.
Key prospective results
Week 24
| Changes in CORALreef Lipids at Week 24 | Mean | 95% CI |
| Primary analysis (observed) | −55.8% | [−60.9 to −50.7] |
| Post-hoc re-analysis (observed) | −59.7% | [−62.3 to −57.1] |
| Pre-readout prediction (pre-registered) | −54.5% | [−56.1 to −53.0] (predictive) |
The predictive interval is entirely contained within the primary 95% CI and lies just above, without overlapping, the post-hoc CI.
Week 52
| Changes in CORALreef Lipids at Week 52 | Mean | 95% CI |
| Primary analysis (observed) | −47.6% | [−52.7 to −42.5] |
| Post-hoc re-analysis (observed) | −52.4% | [−55.1 to −49.7] |
| Pre-readout prediction (pre-registered) | −52.7% | [−54.3 to −51.2] (predictive) |
The predictive interval is entirely contained within the post-hoc 95% CI and touches the lower boundary of the primary 95% CI.
Across both Week 24 and Week 52, Nova’s pre-declared prediction is quantitatively consistent with the clinical data, falling inside at least one of the observed 95% confidence intervals—within the primary CI at Week 24 and within the post-hoc CI at Week 52.
“Delivering a prospective prediction in cardiometabolism aligning with a large Phase 3 program is an important milestone for the field and for Nova,” said Gregoire Boutonnet, co‑CEO of Nova In Silico. “After our two oncology demonstrations in NSCLC, this result shows that our causal AI and digital twins approach can generalize across therapeutic areas to de‑risk programs and inform smarter trial design”
“As an external expert who, with colleagues, contributed to the original validation of Nova’s model, I am very pleased to see these prospective results,” said Bertrand Cariou, MD-PhD (Lipidologist, l’institut du thorax, Nantes, France). “They reinforce once again the body of work that has been published over the past two years and highlight how rigorous in silico evidence can complement and strengthen clinical development.”
Figures
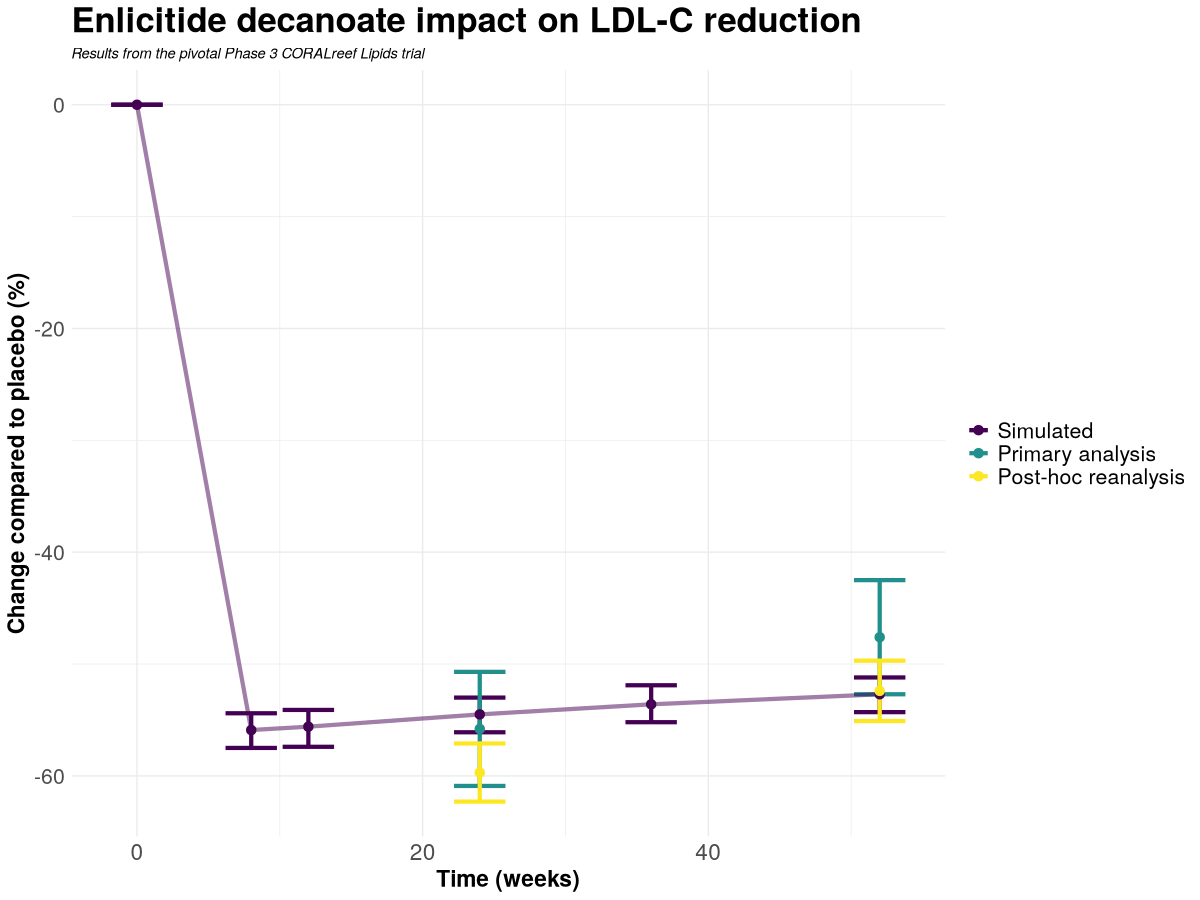
Figure 1. Enlicitide decanoate impact on LDL‑C reduction (change vs placebo, %). The curve shows Nova’s pre‑registered simulation and 95% predictive interval; markers with error bars show the observed Phase 3 outcomes at Weeks 24 and 52 (primary analysis and post‑hoc re‑analysis), as released by Merck.
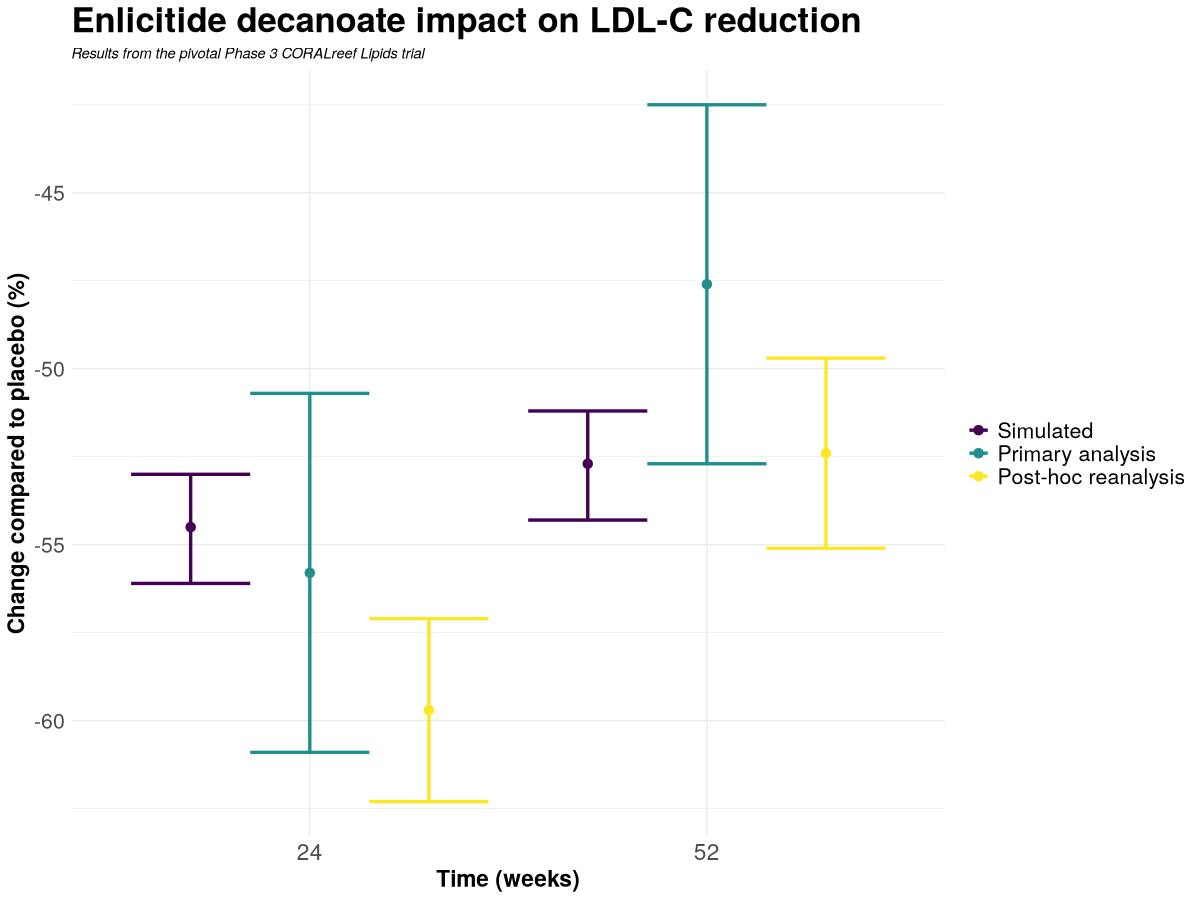
Figure 2. Zoomed view of Weeks 24 and 52. Error bars for observed analyses represent 95% confidence intervals; the simulated intervals are 95% predictive intervals. Both the Week 24 primary analysis and the Week 52 post‑hoc re‑analysis show close agreement with Nova’s pre‑declared predictions.
How the prediction was made
Nova used a mechanistic ASCVD quantitative systems pharmacology (QSP) framework and a trial‑size‑matched virtual patient population calibrated to public Phase 2 information and standard background lipid‑lowering therapies. Using publicly available sources, Nova pre‑specified the Week 24 and Week 52 LDL‑C outcomes and uncertainty (95% predictive percentile intervals) and posted them to Zenodo ahead of the readout: Week 24 −54.5% [−56.1, −53.0]; Week 52 −52.7% [−54.3, −51.2]. The post‑readout comparison confirmed that the observed estimates fell within the pre‑declared predictive intervals for the Week 24 primary analysis and the Week 52 post‑hoc re‑analysis.
A track record of prospective accuracy
This cardiometabolic validation follows Nova’s two prospective NSCLC predictions in 2023, where the company independently anticipated Phase 3 outcomes using its jinkō platform.
About Nova In Silico
Nova In Silico is a health-tech company advancing AI‑enabled, in silico clinical trial simulation. Nova’s jinkō platform combines mechanistic disease models, scalable virtual patient populations, and best‑practice simulation workflows to help pharma industry design smarter trials, reduce development risk, and generate decision‑grade digital evidence—before human studies. Learn more at novainsilico.ai.
Media contact
Gregoire Boutonnet : media@novainsilico.ai
Disclaimer: Enlicitide decanoate (MK‑0616) is an investigational product. Nova In Silico conducted this work independently without any contact with Merck or access to embargoed data; the in silico predictions are research outputs and do not constitute medical claims or regulatory conclusions.
Follow Novadiscovery on LinkedIn and Twitter for more news and information as it becomes available.

